Emory joins NIH-sponsored study for COVID-19 vaccine
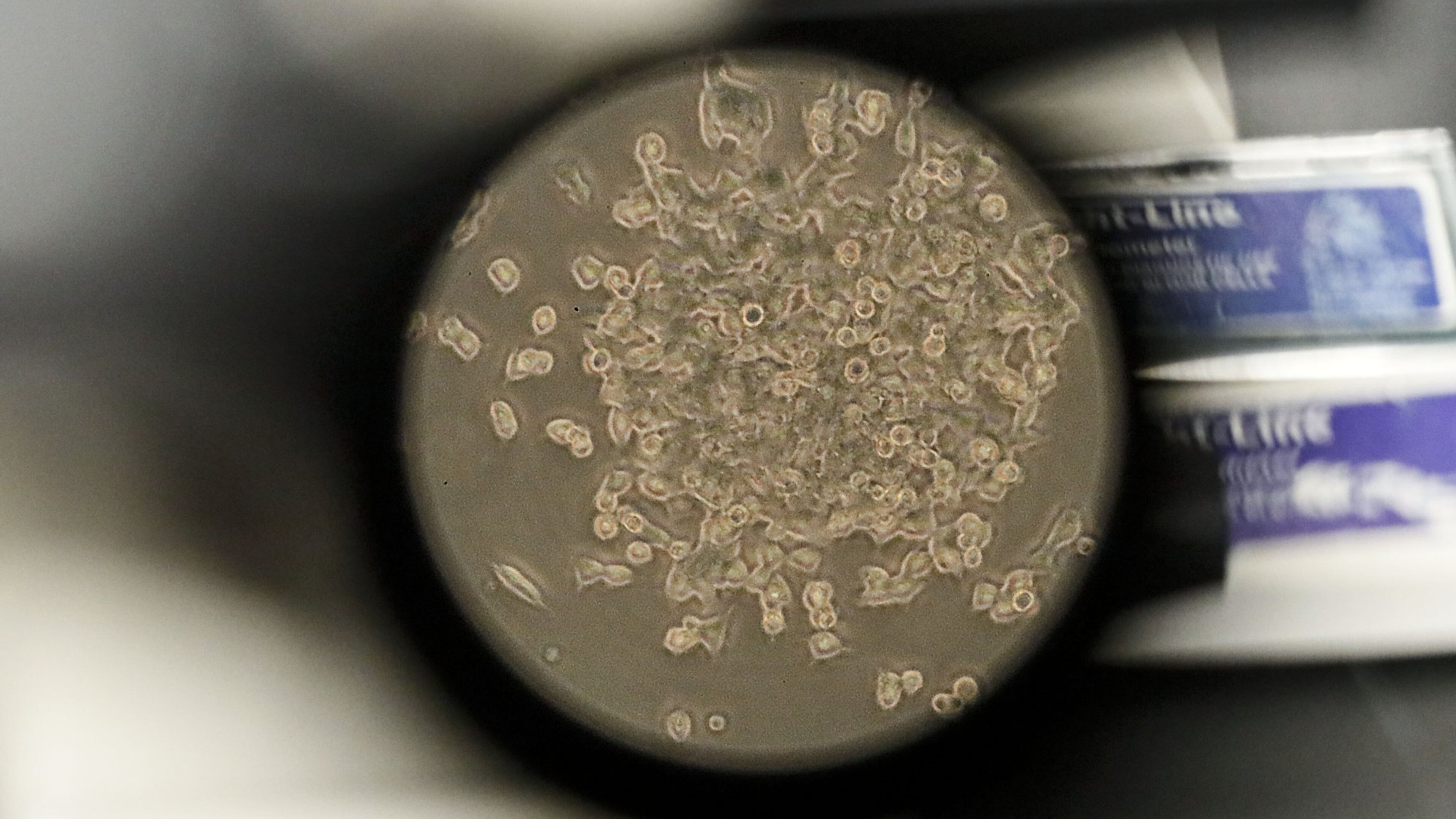
Emory’s Vaccine and Treatment Evaluation unit is participating in a clinical trial to test an experimental vaccine for COVID-19.
>> RELATED: In the race to treat COVID-19, Georgia companies stake a claim
The trial began March 16 at Kaiser Permanente Washington Health Research Institute in Seattle when a 43-year-old mother of two made headlines as the first human to receive the mRNA-1273 vaccine.
Developed by Massachusetts-based Moderna, Inc., the vaccine is based on messenger RNA which signals the body to make a viral protein. It does not contain the virus and cannot cause infection.
» COMPLETE COVERAGE: Coronavirus in Georgia
“The COVID-19 pandemic has caused substantial morbidity and mortality in the US and worldwide along with causing massive social disruption,” said principal investigator, Dr. Evan Anderson in a statement. “The Emory VTEU is proud to contribute to enrolling people into this critical Phase I study evaluating the first vaccine candidate against COVID-19.”
» DASHBOARD: Real-time stats and charts of coronavirus in Georgia
The trial aims to enroll about 45 participants in Seattle and Atlanta. Eligible participants must be ages 18 - 55 and cannot have chronic diseases or health conditions that affect the immune system nor can they be taking immunosuppressive medications.
Earlier this month, the chief medical officer for Moderna, Tal Zaks, told STAT that scientists at NIH are "working on nonclinical research in parallel"indicating a departure from the traditional model of testing a vaccine to see if it prevents infection in animals before testing it in humans.
Emory has been part of NIAID’s research consortium since 2007.

