Third shots authorized for Georgians with weakened immunity

Georgians who are being treated for cancer, have had organ transplants or have advanced HIV or AIDS are now eligible to receive a third dose of the Pfizer or Moderna vaccines to increase their protection against COVID-19.
The Centers for Disease Control and Prevention’s independent panel of vaccine experts on Friday signed off on the additional shot for those with moderate to severe immunity impairments, citing their increased risk of severe disease.
For most people, the CDC says the COVID-19 vaccines do what they were designed to do: Keep people out of hospitals and alive. But research indicates two doses of the Pfizer or Moderna vaccines didn’t result in an adequate immune response for some whose immune systems are suppressed by medications such as steroids or cancer drugs. The shots also weren’t enough for those whose immune system doesn’t function adequately, the CDC’s Advisory Committee on Immunization Practices said.
“What we know from CDC data is that 40% to 45% of breakthrough infections of those that are vaccinated and resulted in hospitalization came from the 2.7% of the adult population that is classified as immune compromised,” Dr. Joshua Wyche, pharmacy manager for ambulatory and oncology services at Augusta University Health, told The Atlanta Journal-Constitution.
However, some physicians told the CDC panel that they are concerned about the risk to others with chronic health conditions, such as diabetes, given the escalating threat of variant strains.
Others are calling for additional shots for the elderly, noting that immune systems weaken as people age. According to the CDC, 74% of those with breakthrough cases of COVID-19 after being fully vaccinated were age 65 or older.
Public health experts view the decision to authorize the shots for those with moderate to severe immunity impairments as a possible first step toward eventually authorizing third shots for the general population.
“What’s going to be the big issue down the road is waning immunity — people who’ve been vaccinated who aren’t immunocompromised in any way,” said Dr. Michael Eriksen, founding dean of Georgia State’s School of Public Health.
Thursday, the Food and Drug Administration expanded emergency use authorization of the Pfizer-BioNTech and Moderna vaccines to allow the additional dose for those with compromised immune systems.
Before Friday’s unanimous vote by the vaccine advisory committee, there was strong support of the FDA’s latest stance among its members.
“I really think this is overdue, but we’re working as fast as we can,” ACIP member Lynn Bahta, a nurse and clinical consultant with the Minnesota Department of Public Health, said just prior to the committee’s vote.
As a result of the vote, those age 12 and older with weakened immune systems will be eligible to receive a third dose of the Pfizer vaccine, while those 18 and older will be able to receive an additional dose of the Moderna vaccine.
Those who received the one-dose J&J vaccine are not included in the expanded emergency use authorization, however. That’s because of a lack of supporting data at this time, the panel’s experts said.
Third dose criteria
The committee’s discussion centered on two important issues: whether children should be allowed to get third doses and how “immunocompromised” would be defined.
In preparation for Friday’s discussion, the committee’s workgroup had focused on evidence of the vaccine’s effectiveness with immunocompromised adults. But the FDA’s move to expand emergency use authorization of the Pfizer vaccine for those as young as 12 years of age prompted panel members Friday to discuss at length whether additional doses should be allowed for younger ages.
The consensus among physicians who serve young patients with cancer and other serious illnesses is to make a third dose available to them. “They all supported a lower age because the lowest age we have right now is 12,” said Dr. Camille Nelson Kotton, ACIP member and an associate professor at Harvard Medical School.
No vaccine has yet been authorized for those younger than 12.
Physicians also asked the committee for a clearer definition who would qualify for the third shots. Its definition of immunocompromised included those on active treatment for solid-tumor malignancies and cancers that affect the blood, bone marrow and lymph nodes, as well as those who have received solid-organ transplants and are taking immunosuppressive therapy.
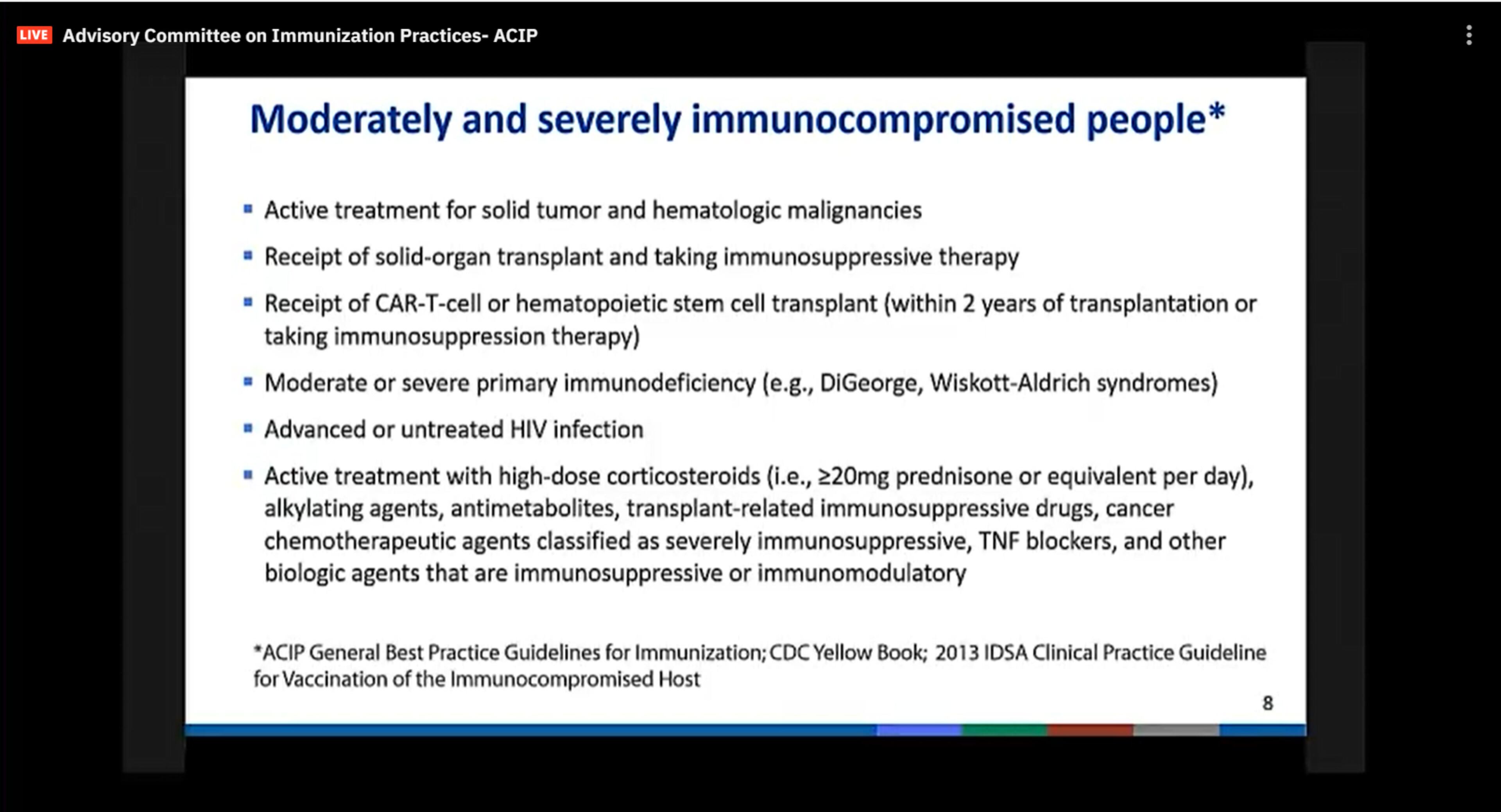
Also included are recipients of CAR-T-cell therapies or bone marrow transplants, those with moderate or severe primary immunodeficiency, as well as persons with advanced or untreated HIV infection. Lastly, persons undergoing high-dose steroid treatments, which are immunosuppressive, also are eligible for the additional dose, the panel said.
Others with chronic health conditions are awaiting word on when they will be eligible.
Andy Lipman, a 47-year-old Sandy Springs resident who has cystic fibrosis, is among them. Those with cystic fibrosis aren’t considered immune compromised unless they have had an organ transplant. Lipman has been spared that, but he notes that the inherited disorder causes severe damage to the lungs, digestive system and other organs.
“I probably need to get the booster as soon as possible,” he said. “My physicians are looking into it.”
Waning immunity?
Others have been clamoring for additional vaccine protection, as hospitals have been hit with a fourth wave of infected patients.
Some haven’t waited for the federal government to authorize the additional shots. In recent weeks, an estimated 1.14 million people sought out third doses of either the Pfizer of Moderna vaccines on their own, said Dr. Kathleen Dooling of the CDC at Friday’s meeting. Almost 91,000 received an additional dose of the J&J vaccine, she said.
Also fueling the concern are higher than anticipated breakthrough cases among the fully vaccinated as the highly contagious delta variant has spread. So far, such cases are rare. Out of the 4.9 million fully vaccinated Georgians, 13,332 have tested positive for the coronavirus as of Aug. 10, according to the Georgia Department of Public Health. Of those, 198 have been hospitalized for COVID-19, and 105 have died.
However, while vaccinated people are far less likely to experience serious illness, they still may spread the infection to others, experts say.
Dr. Felipe Lobelo, an epidemiologist for Kaiser Permanente of Georgia, said he suspects all vaccinated people become more vulnerable to infections with the passing months, based on studies from Israel and European nations that have already weathered their own delta surges.
He is concerned that the CDC is tracking only the breakthrough cases that result in hospitalization or death. The agency should be tracking all breakthroughs, he said.
“I will not be surprised if in a few weeks, as more delta cases accumulate and as we have better data from cohort studies and from other studies that the CDC is doing, to hear that the vaccine effectiveness actually is lower now and that the percentage of breakthrough cases is higher,” said Lobelo, a former epidemic intelligence service officer for the CDC.

By early September, the FDA has said it may establish a national strategy on booster shots.
There are global concerns, though, about authorizing additional shots for the general population when people in other countries have yet to get first shots. Surges in largely unvaccinated nations can give rise to more dangerous new variants, experts warn.
“Should countries like the U.S. and the U.K. and France and Germany and Italy be giving third shots when people in other countries haven’t gotten their first shot yet?” Dr. Eriksen, of GSU, said.
Others note that the large number of unvaccinated Americans also puts the country at risk.
“By having more cases and ... an explosion of infections in the South right now, we are giving the virus essentially a leg up to potentially mutate and escape the immunity that the vaccines are providing,” Lobelo said.
The state’s vaccination rate has slowly ticked up over the past month and a half, with the percentage of Georgians fully vaccinated rising from 38% on July 1 to 41% on Friday, according to the Georgia Department of Public Health. But that’s not enough to prevent more hospitalizations and deaths when the delta surge peaks, which is expected to happen by early September.
With the rampant vaccine hesitancy in Georgia, shots are readily available for those now eligible for the third doses. Georgia’s supply of vaccine far outweighs demand, said DPH spokeswoman Nancy Nydam. “Should there be a need, we can also order more from the feds,” she said in an email to the AJC.
Getting a third dose
About 7 million American adults are immunocompromised and at risk of severe COVID-19 infections. To better protect them, the CDC’s Advisory Committee on Immunization Practices authorized an extra dose of Pfizer-BioNTech or Moderna vaccines, but noted these caveats:
♦The third dose should be administered no sooner than 28 days after completion of the second dose.
♦A prescription is not required to get the extra dose.
♦Clinicians should try to use the same type of vaccine as the original series.
♦Vulnerable patients who receive a third dose should still wear a mask, practice social distancing, and avoid crowds and poorly ventilated indoor spaces.


