Alpharetta company recalls thyroid drug

Alpharetta-based Acella Pharmaceuticals is voluntarily recalling 13 lots of a thyroid medication after receiving two reports that patients who took the drug suffered adverse effects, the FDA said. Following those reports, the company tested the drug NP Thyroid and found it was super potent, with up to 115% of the labeled amount of a hormone, liothyronine, used to treat an under-active thyroid.
Patients who take the superpotent tablets could suffer fatigue, muscle weakness, chest pain, a rapid heart beat or other disturbances. Those who are pregnant could suffer miscarriages or impaired fetal development, the federal agency said.
NP Thyroid was distributed nationwide in 30 mg, 60 mg and 90 mg tablets. The recalled products are in 100-count bottles and have expiration dates or July, August, November or December of this year. A list of the lots affected can be found on the Food and Drug Administration website. Consumers can also call Acella at 1-800-541-4802 or by email at recall@acellapharma.com.
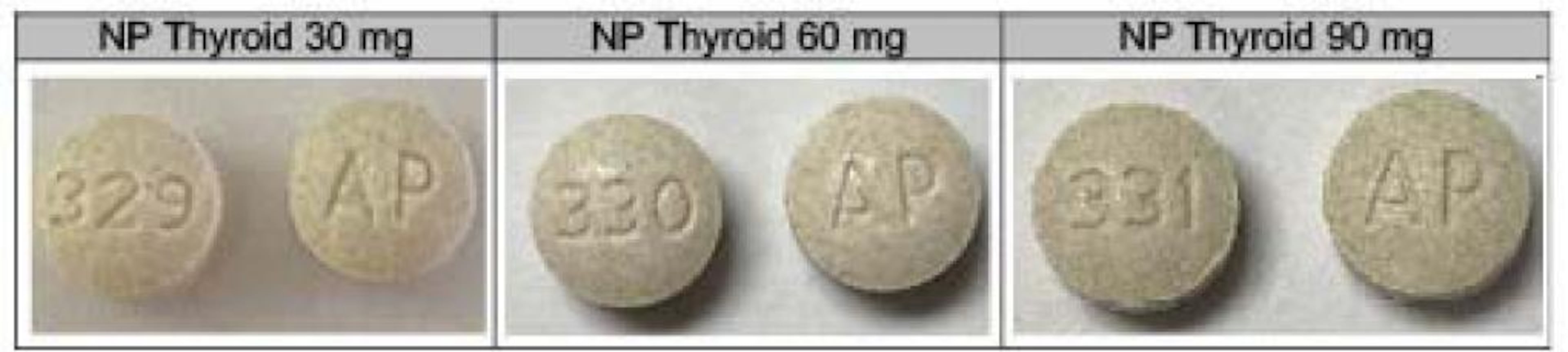
The company is notifying its wholesalers to stop distributing the drugs. But the FDA said that patients who are currently taking NP Thyroid from the lots being recalled should not stop taking them without contacting their health care providers for guidance and a replacement prescription.
Acella is a specialty pharmaceutical company founded in 2007.


