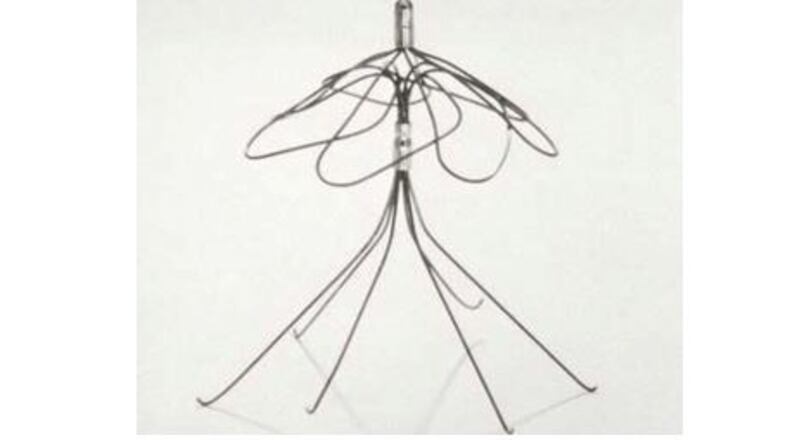
C.R. Bard Inc. was bought by Becton Dickinson, or B.D., last year. A spokesman for the companies, Troy Kirkpatrick, provided this statement to The Atlanta Journal-Constitution.
C. R. Bard Inc. (now part of B.D.) fully complies with all regulations from the U.S. Food and Drug Administration surrounding safety and efficacy testing of our products. Our IVC filter products are cleared for use by the FDA and continue to offer life-saving benefits that have helped tens of thousands of patients for over a decade.
Prior to filing for 510(K) clearance with the FDA, Bard conducted a large variety of testing including clot trapping efficiency tests, weld integrity tests, fatigue tests, corrosion tests, biocompatibility tests, hook strength tests, radial strength tests, migration resistance tests, recovery tests, as well as tests that determined the effect the IVC filter had on tissue. The company also has conducted three clinical studies covering nearly 360 patients and is currently participating in an additional clinical study regarding filters for 2,500 patients.
Any implantable medical device carries inherent risks, but also provides clinical benefits that outweigh those risks. We provide information about both the risks and benefits in order that physicians, in consultation with their patients, can determine whether those benefits outweigh the risks in a particular instance. While we understand that some patients required additional interventions or treatment some years after implantation of a filter, it is important to note that the filter helped to protect them from the life-threatening blood clots from which they suffered, and which typically could not be treated with medication.
The Latest
Featured